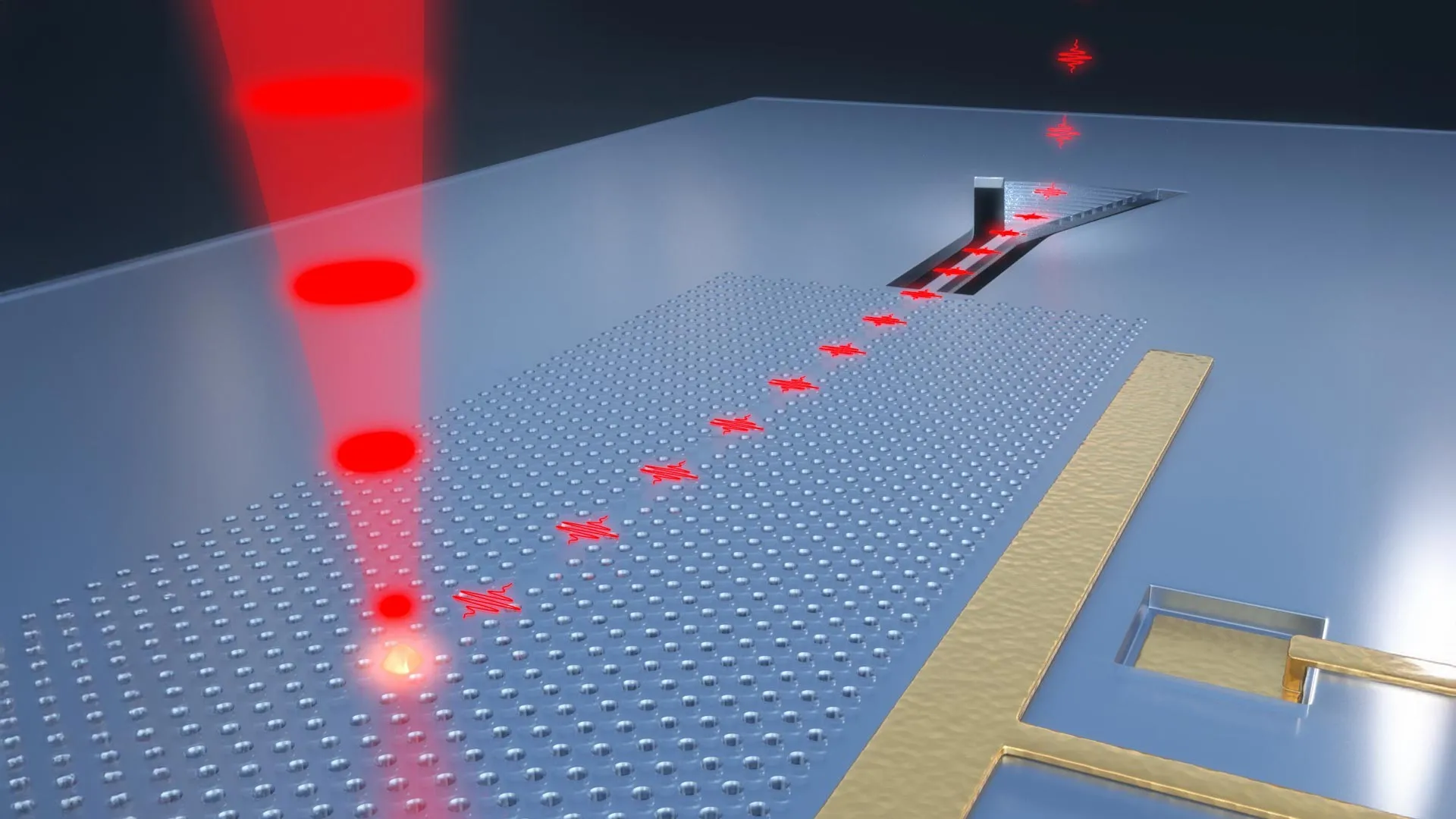
The transformative potential of strontium titanate lies in its remarkable electro-optic and piezoelectric properties, which are dramatically amplified at cryogenic temperatures. As explained by Jelena Vuckovic, the study’s senior author and a distinguished professor of electrical engineering at Stanford, "Strontium titanate has electro-optic effects 40 times stronger than the most-used electro-optic material today. But it also works at cryogenic temperatures, which is beneficial for building quantum transducers and switches that are current bottlenecks in quantum technologies." This amplified electro-optic effect means that when an electric field is applied, STO’s optical and mechanical characteristics undergo substantial and highly controllable shifts. This tunability is crucial for scientists who need to precisely manipulate light, adjusting its frequency, intensity, phase, and direction in ways that are simply not possible with existing materials. This unparalleled versatility opens the door to the development of entirely novel types of low-temperature devices, enabling functionalities previously confined to theoretical speculation.
Beyond its optical prowess, STO’s piezoelectric nature—its ability to physically expand and contract in response to electric fields—makes it an ideal candidate for the development of new electromechanical components that are engineered to function with peak efficiency in extreme cold. The researchers highlight that these capabilities are particularly valuable for applications in the harsh, vacuum environment of space or within the cryogenic fuel systems of advanced rockets, where reliable performance under extreme thermal conditions is paramount. Christopher Anderson, a co-first author of the study and now a faculty member at the University of Illinois, Urbana-Champaign, further emphasized this point: "At low temperature, not only is strontium titanate the most electrically tunable optical material we know of, but it’s also the most piezoelectrically tunable material." This dual enhancement of both optical and mechanical tunability at cryogenic temperatures positions STO as a truly exceptional material for a wide array of demanding applications.
Perhaps one of the most astonishing aspects of this discovery is that strontium titanate, the material exhibiting these extraordinary capabilities, is not a newly synthesized compound. It is a substance that has been extensively studied for decades, is readily available, and is remarkably inexpensive. Giovanni Scuri, a co-first author and a postdoctoral scholar in Vuckovic’s lab, pointed out this surprising practicality: "STO is not particularly special. It’s not rare. It’s not expensive." In fact, Scuri elaborated, STO has historically found use as a diamond substitute in jewelry or as a substrate for growing more valuable materials. Its widespread availability and low cost, coupled with its textbook understanding, make its exceptional cryogenic performance even more remarkable. The decision to investigate STO’s potential in this context was driven by a deep understanding of the fundamental characteristics that contribute to a material’s high tunability. "We knew what ingredients we needed to make a highly tunable material," Anderson explained. "We found those ingredients already existed in nature, and we simply used them in a new recipe. STO was the obvious choice." The outcome, he added, was astonishing: "When we tried it, surprisingly, it matched our expectations perfectly." Scuri further noted that the theoretical framework developed by the team for this research could serve as a blueprint for identifying or enhancing other nonlinear materials for various operating conditions, extending the impact of their work beyond STO itself.
The experimental results at cryogenic temperatures were nothing short of revelatory. When tested at a frigid 5 Kelvin (-450°F), STO’s performance far exceeded even the researchers’ ambitious expectations. Its nonlinear optical response was a staggering 20 times greater than that of lithium niobate, the current industry standard for nonlinear optical materials, and nearly triple that of barium titanate, which had previously held the benchmark for cryogenic performance. To push these already exceptional properties even further, the team embarked on a sophisticated modification of the crystal structure. By replacing a specific fraction of oxygen atoms with their heavier isotopic counterparts, they were able to nudge STO closer to a state known as quantum criticality. This strategic alteration resulted in an even more pronounced increase in tunability. "By adding just two neutrons to exactly 33 percent of the oxygen atoms in the material, the resulting tunability increased by a factor of four," Anderson proudly stated. "We precisely tuned our recipe to get the best possible performance." This meticulous tuning demonstrates a profound level of control and understanding of the material’s quantum mechanical behavior.
The practical advantages of STO extend beyond its unparalleled performance characteristics. According to the research team, its suitability for integration into existing manufacturing processes makes it particularly appealing to engineers. STO can be synthesized, structurally modified, and fabricated at wafer scale using the very same semiconductor equipment that is standard in the industry today. This compatibility significantly streamlines the development and production of next-generation quantum devices, particularly laser-based switches that are essential for controlling and transmitting quantum information. The significance of this discovery has not gone unnoticed by industry leaders. The research received partial funding from major players in the quantum computing arena, including Samsung Electronics and Google’s quantum computing division, both of whom are actively seeking advanced materials to propel their quantum hardware development. The team’s immediate next objective is to translate these remarkable material properties into fully functional cryogenic devices, demonstrating the real-world applicability of their findings. "We found this material on the shelf," Anderson concluded, summarizing the extraordinary journey. "We used it and it was amazing. We understood why it was good. Then the cherry on the top — we knew how to do better, added that special sauce, and we made the world’s best material for these applications. It’s a great story." The study’s broader support network included a Vannevar Bush Faculty Fellowship from the U.S. Department of Defense and the Department of Energy’s Q-NEXT program, underscoring the national importance of this research. Contributors from various institutions, including Aaron Chan and Lu Li from the University of Michigan, Sungjun Eun, Alexander D. White, Geun Ho Ahn, Amir Safavi-Naeini, and Kasper Van Gasse from Stanford’s E. L. Ginzton Laboratory, and Christine Jilly from the Stanford Nano Shared Facilities, played crucial roles in this monumental achievement.