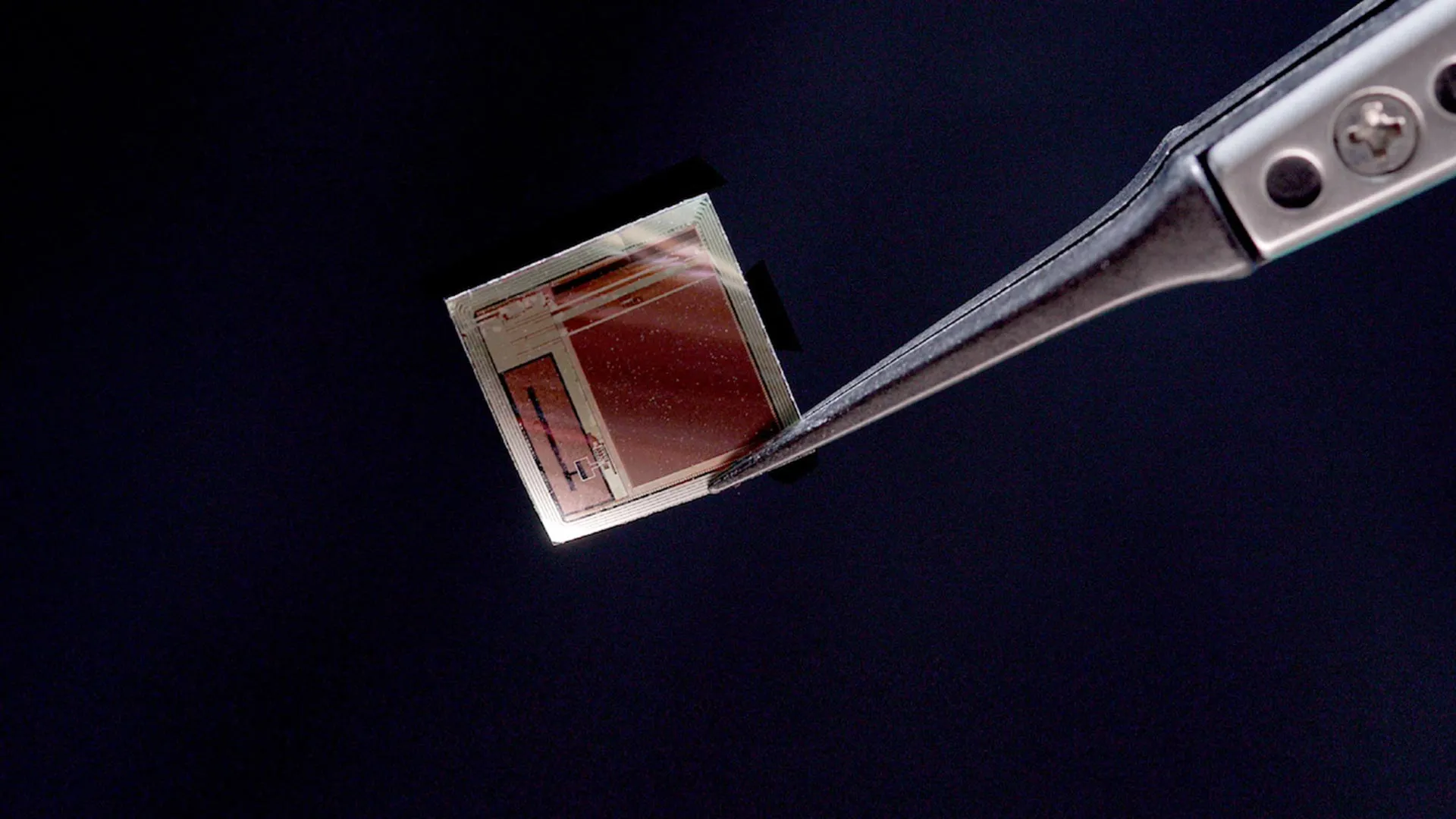
The remarkable promise of BISC stems from its exceptionally compact design, a stark contrast to existing bulky implantable systems, coupled with its extraordinary data transmission capabilities. At its core, BISC is a single, ultra-thin silicon chip designed to create a seamless, wireless, and high-bandwidth connection between the brain and external computing systems. This innovative approach dramatically redefines the limitations of current BCI technology.
A pivotal study, published on December 8th in the esteemed journal Nature Electronics, meticulously details the intricate architecture of BISC. The system comprises the core chip-based implant, a discreet wearable "relay station," and the sophisticated software required to operate the entire platform. Ken Shepard, a distinguished figure at Columbia University holding professorships in Electrical Engineering, Biomedical Engineering, and Neurological Sciences, and a senior author who spearheaded the engineering efforts, highlighted the radical departure from conventional implants. "Most implantable systems are built around a canister of electronics that occupies enormous volumes of space inside the body," Shepard explained. "Our implant is a single integrated circuit chip that is so thin that it can slide into the space between the brain and the skull, resting on the brain like a piece of wet tissue paper." This feather-light and incredibly thin profile minimizes surgical invasiveness and the potential for tissue damage, a critical factor for long-term implantable devices.
The transformative potential of BISC lies in its ability to convert the delicate surface of the cortex into a high-bandwidth interface. Shepard collaborated closely with Andreas S. Tolias, PhD, a senior and co-corresponding author, professor at the Byers Eye Institute at Stanford University, and co-founding director of the Enigma Project. Tolias’s profound expertise in training artificial intelligence (AI) systems on vast neural datasets, including those acquired through BISC, proved instrumental in evaluating the implant’s capacity to decode brain activity with unprecedented accuracy. "BISC turns the cortical surface into an effective portal, delivering high-bandwidth, minimally invasive read-write communication with AI and external devices," Tolias stated. "Its single-chip scalability paves the way for adaptive neuroprosthetics and brain-AI interfaces to treat many neuropsychiatric disorders, such as epilepsy." This dual capability of both reading and writing neural information opens up exciting possibilities for both understanding brain function and actively modulating it for therapeutic purposes.
The clinical dimension of this groundbreaking research was spearheaded by Dr. Brett Youngerman, an assistant professor of neurological surgery at Columbia University and a neurosurgeon at NewYork-Presbyterian/Columbia University Irving Medical Center. Dr. Youngerman underscored the revolutionary impact of BISC on managing neurological conditions. "This high-resolution, high-data-throughput device has the potential to revolutionize the management of neurological conditions from epilepsy to paralysis," he asserted. Recognizing the immense potential, Dr. Youngerman, in conjunction with Shepard and Dr. Catherine Schevon, an epilepsy neurologist at NewYork-Presbyterian/Columbia, has already secured a prestigious National Institutes of Health grant to explore BISC’s efficacy in treating drug-resistant epilepsy. "The key to effective brain-computer interface devices is to maximize the information flow to and from the brain, while making the device as minimally invasive in its surgical implantation as possible. BISC surpasses previous technology on both fronts," Youngerman added, emphasizing the device’s dual strengths in information capacity and surgical gentleness.
Shepard further elaborated on the technological underpinnings, drawing a parallel to the exponential growth in computing power. "Semiconductor technology has made this possible, allowing the computing power of room-sized computers to now fit in your pocket," he remarked. "We are now doing the same for medical implantables, allowing complex electronics to exist in the body while taking up almost no space." This analogy effectively conveys the scale of miniaturization and integration achieved with BISC.
Next-Generation BCI Engineering: A Revolution in Design
The fundamental difference in BISC’s engineering lies in its integrated design, a departure from the multi-component architectures of current medical-grade BCIs. Traditionally, these systems rely on separate microelectronic components such as amplifiers, data converters, and radio transmitters. These disparate parts necessitate a relatively large implanted canister, requiring either removal of a portion of the skull or implantation in another body cavity, with wires extending to the brain. BISC, however, consolidates all these functionalities onto a single complementary metal-oxide-semiconductor (CMOS) integrated circuit.
This innovative chip has been thinned to an astonishing 50 micrometers, occupying less than 1/1000th the volume of a standard implant. With a total volume of approximately 3 cubic millimeters, the flexible chip is designed to conform to the brain’s intricate surface. This micro-electrocorticography (µECoG) device is equipped with an impressive 65,536 electrodes, enabling 1,024 recording channels and 1,024 stimulation channels. Crucially, the chip’s fabrication utilizes established semiconductor industry manufacturing methods, ensuring its suitability for large-scale, cost-effective production.
The integrated design of the BISC chip is a marvel of modern engineering. It seamlessly incorporates a radio transceiver, a wireless power circuit, digital control electronics, power management systems, data converters, and the analog components essential for both recording neural activity and delivering targeted stimulation. The external relay station plays a vital role, providing power and facilitating data communication through a custom ultrawideband radio link that achieves a remarkable throughput of 100 megabits per second (Mbps). This is an order of magnitude, at least 100 times higher, than any other wireless BCI currently available. Operating as a standard 802.11 WiFi device, the relay station acts as a bridge, effortlessly connecting the implant to any compatible computer.
BISC is not merely a hardware innovation; it also features its own proprietary instruction set and a comprehensive software environment, effectively creating a specialized computing system tailored for brain interfaces. The high-bandwidth recording capabilities demonstrated by this study are crucial for processing brain signals using advanced machine-learning and deep-learning algorithms. These sophisticated algorithms can then interpret complex intentions, perceptual experiences, and nuanced brain states with remarkable accuracy. "By integrating everything on one piece of silicon, we’ve shown how brain interfaces can become smaller, safer, and dramatically more powerful," Shepard emphasized, highlighting the synergistic benefits of this integrated approach.
Advanced Semiconductor Fabrication: The Backbone of Innovation
The advanced capabilities of the BISC implant are made possible by its fabrication using TSMC’s 0.13-micrometer Bipolar-CMOS-DMOS (BCD) technology. This cutting-edge fabrication method represents a significant advancement in semiconductor manufacturing, as it integrates three distinct semiconductor technologies onto a single chip. This allows for the efficient co-existence and interaction of digital logic (from CMOS), high-current and high-voltage analog functions (from bipolar and DMOS transistors), and robust power devices (from DMOS). This sophisticated integration is absolutely critical for achieving the high performance and miniaturization required for BISC.
Moving From the Lab Toward Clinical Use: Bridging the Gap
The transition of BISC from the laboratory to real-world clinical application has been a carefully orchestrated process. Shepard’s team has forged a strong partnership with Dr. Youngerman at NewYork-Presbyterian/Columbia University Irving Medical Center to refine surgical procedures for the safe implantation of the ultra-thin device. Preclinical studies in animal models have yielded promising results, demonstrating the device’s ability to produce high-quality, stable neural recordings. Short-term intraoperative studies in human patients are already underway, marking a critical step towards broader clinical adoption.
"These initial studies give us invaluable data about how the device performs in a real surgical setting," Youngerman commented, emphasizing the real-world validation of the technology. "The implants can be inserted through a minimally invasive incision in the skull and slid directly onto the surface of the brain in the subdural space. The paper-thin form factor and lack of brain-penetrating electrodes or wires tethering the implant to the skull minimize tissue reactivity and signal degradation over time." This description highlights the advantages of BISC’s non-invasive implantation and its minimal impact on delicate brain tissue, factors that are crucial for long-term patient safety and device efficacy.
Extensive preclinical work, focusing on the motor and visual cortices, was conducted in collaboration with Dr. Tolias and Bijan Pesaran, a professor of neurosurgery at the University of Pennsylvania, both recognized luminaries in the fields of computational and systems neuroscience. Their contributions have been vital in understanding and validating the functional capabilities of BISC in complex neural circuits. "The extreme miniaturization by BISC is very exciting as a platform for new generations of implantable technologies that also interface with the brain with other modalities such as light and sound," Pesaran remarked, pointing towards future avenues of research and development.
The development of BISC was a significant undertaking, supported by the Neural Engineering System Design program of the Defense Advanced Research Projects Agency (DARPA). This initiative leveraged Columbia University’s deep-rooted expertise in microelectronics, the advanced neuroscience programs at Stanford and Penn, and the cutting-edge surgical capabilities of NewYork-Presbyterian/Columbia University Irving Medical Center, creating a truly interdisciplinary and collaborative environment.
Commercial Development and Future AI Integration: Charting the Path Forward
To accelerate the journey of BISC from research to widespread practical use, researchers at Columbia and Stanford have established Kampto Neurotech. This promising startup was founded by Dr. Nanyu Zeng, a Columbia electrical engineering alumnus and one of the lead engineers on the BISC project. Kampto Neurotech is actively producing research-ready versions of the chip and diligently working to secure the necessary funding to prepare the system for clinical trials and eventual use in human patients. "This is a fundamentally different way of building BCI devices," Zeng stated, underscoring the disruptive nature of BISC. "In this way, BISC has technological capabilities that exceed those of competing devices by many orders of magnitude."
As artificial intelligence (AI) continues its relentless advancement, BCIs are increasingly recognized for their dual potential: not only for restoring lost abilities in individuals suffering from neurological disorders but also for potentially enhancing normal human function through direct brain-to-computer communication. The synergy between advanced BCIs and AI is becoming increasingly apparent.
"By combining ultra-high resolution neural recording with fully wireless operation, and pairing that with advanced decoding and stimulation algorithms, we are moving toward a future where the brain and AI systems can interact seamlessly — not just for research, but for human benefit," Shepard concluded, painting an optimistic vision for the future. "This could change how we treat brain disorders, how we interface with machines, and ultimately how humans engage with AI." This ambitious outlook suggests that BISC and similar technologies are not merely incremental improvements but represent a fundamental shift in our relationship with both our own biology and the rapidly evolving landscape of artificial intelligence.