Imagine a future where factories churn out novel materials and intricate chemical compounds with unprecedented speed, at significantly reduced costs, and with streamlined production processes. Envision your personal laptop effortlessly crunching complex data in mere seconds, or a supercomputer demonstrating the learning and adaptive capabilities of the human brain. These transformative possibilities are intrinsically linked to a fundamental factor: the enigmatic behavior of electrons within materials. Now, researchers at Auburn University have achieved a monumental breakthrough, developing a groundbreaking class of materials that empower scientists with exquisite control over these minuscule charged particles. Their pioneering findings, meticulously detailed in the esteemed journal ACS Materials Letters, illuminate how the team has engineered adjustable coupling between isolated-metal molecular complexes, specifically dubbed solvated electron precursors. In these revolutionary materials, electrons are liberated from their atomic shackles, no longer tethered to specific nuclei but instead flowing freely through expansive interstitial spaces. This fundamental shift in electron behavior is poised to catalyze a new era of technological innovation, potentially ushering in the next quantum revolution.
Electrons, the unsung heroes of the atomic world, are undeniably central to an astonishing array of chemical and technological processes. They orchestrate energy transfer, forge the bonds that hold matter together, and conduct electricity, forming the bedrock of both sophisticated chemical synthesis and the ubiquitous modern electronics that define our daily lives. Within the intricate dance of chemical reactions, electrons are the linchpins of redox processes, the architects of new bond formations, and the catalysts that accelerate transformations. In the realm of technology, the ability to precisely manage how electrons move, interact, and are channeled underpins everything from the intricate circuits of our smartphones and the sophisticated algorithms of artificial intelligence systems to the energy-harvesting mechanisms of solar cells and the nascent power of quantum computers. Traditionally, electrons have been largely confined to the atomic orbitals of specific atoms, a limitation that has inherently restricted their full potential for diverse applications. However, a special class of materials known as "electrides" offers a radical departure from this norm. In electrides, electrons exhibit a remarkable independence, moving freely and delocalized, thereby unlocking a vista of extraordinary new capabilities that were previously confined to theoretical speculation.
Dr. Evangelos Miliordos, an Associate Professor of Chemistry at Auburn University and the senior author of this seminal study, which was heavily reliant on sophisticated computational modeling, enthusiastically explains the profound implications of this research: "By mastering the art of controlling these free-roaming electrons, we are no longer bound by the limitations imposed by nature. We can now engineer materials that possess functionalities and perform tasks that were previously unimaginable." This sentiment underscores the paradigm shift that the Auburn team’s work represents.
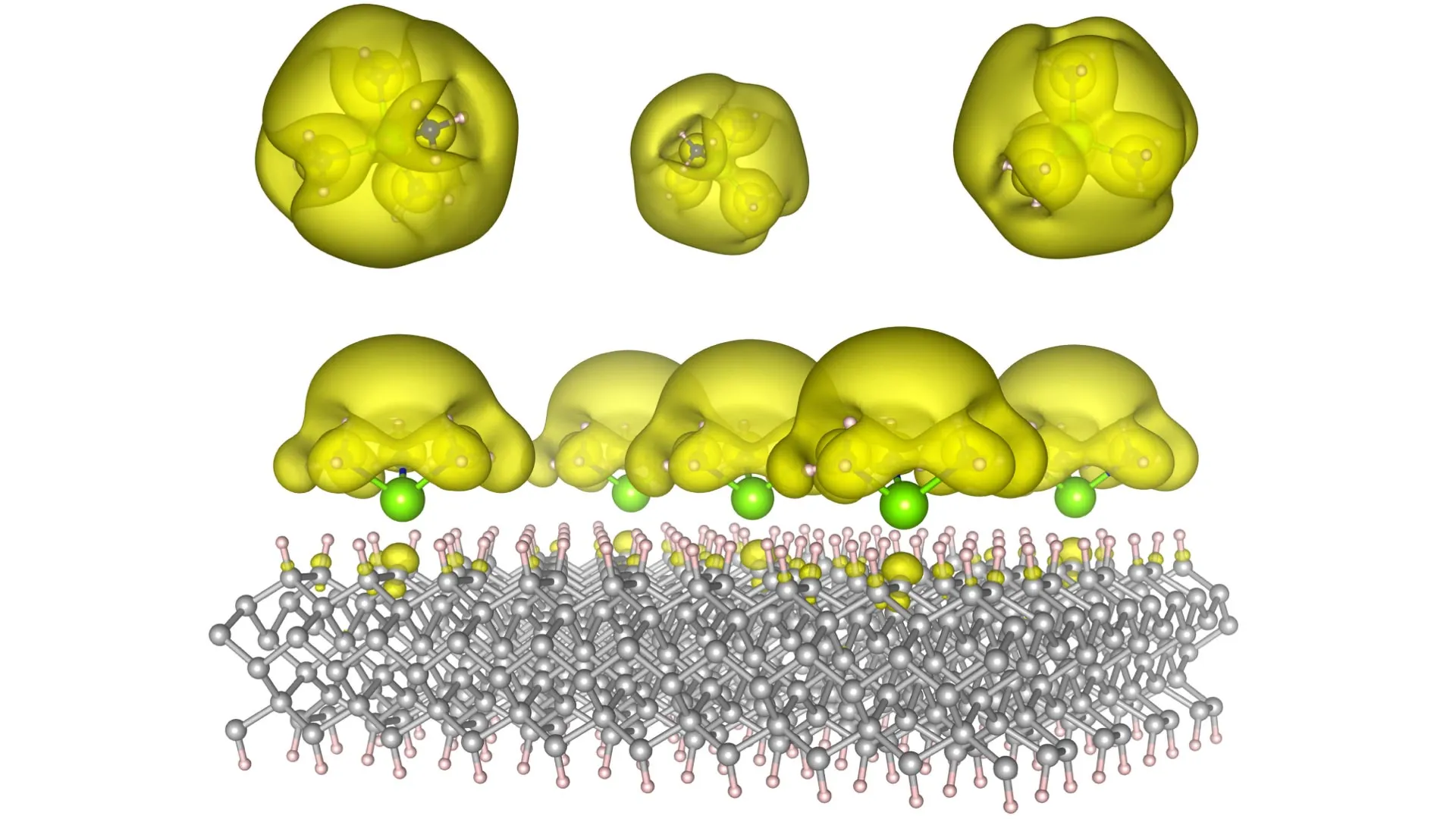
To achieve this unprecedented level of control, the Auburn researchers ingeniously devised innovative material architectures they termed "Surface Immobilized Electrides." This was accomplished by anchoring solvated electron precursors onto robust and stable surfaces, such as the atomically precise structures of diamond and the widely used semiconductor silicon carbide. This strategic immobilization confers a dual benefit: it renders the electronic characteristics of the resulting electrides both exceptionally durable and remarkably tunable. The team discovered that by meticulously adjusting the spatial arrangement of the molecular precursors on the surface, they could dictate the behavior of the delocalized electrons. They can be coaxed to aggregate into isolated "islands," which, at the quantum level, behave analogously to the fundamental units of quantum information – qubits – thereby paving the way for the development of advanced quantum computing architectures. Alternatively, these electrons can be induced to spread out into extended, continuous "seas," a configuration that significantly enhances their capacity to facilitate and accelerate complex chemical reactions, acting as potent catalysts.
This remarkable versatility is the very essence of why this discovery possesses such transformative potential. One manifestation of this breakthrough could directly lead to the development of immensely powerful quantum computers. Such machines would be capable of tackling computational problems that currently lie far beyond the reach of even the most advanced supercomputers, potentially revolutionizing fields like drug discovery, materials science, and fundamental physics. Concurrently, another application of these tunable electrides could provide the foundational basis for the creation of cutting-edge catalysts. These catalysts would dramatically accelerate essential chemical reactions, promising to revolutionize the industrial production of everything from clean fuels and life-saving pharmaceuticals to advanced industrial materials, thereby fostering greater sustainability and efficiency across numerous sectors.
Dr. Marcelo Kuroda, an Associate Professor of Physics at Auburn, highlights the growing imperative for novel materials: "As our society relentlessly pushes the boundaries of current technological capabilities, the demand for new and innovative kinds of materials is experiencing an explosive surge. Our research offers a compelling new pathway towards the creation of materials that not only open up exciting avenues for fundamental investigations into the intricate interactions within matter but also hold immense promise for practical, real-world applications." This statement emphasizes the dual impact of the research, bridging the gap between fundamental scientific inquiry and tangible technological advancement.
Previous iterations of electrides, while demonstrating the potential of delocalized electrons, often suffered from inherent instability and proved exceedingly difficult to produce at scale. The Auburn team’s innovative approach of depositing these electrides directly onto solid surfaces has effectively surmounted these critical barriers. They have successfully proposed a versatile family of material structures that are no longer confined to the realm of theoretical models but are now poised to transition into tangible, functional devices. Dr. Konstantin Klyukin, an Assistant Professor of Materials Engineering at Auburn, articulates the profound significance of this development: "This is fundamentally groundbreaking science, but its implications are remarkably concrete. We are talking about technologies that have the potential to fundamentally alter how we process information and how we manufacture the products that shape our world."
The intellectual heavy lifting behind this groundbreaking theoretical study was a testament to interdisciplinary collaboration, with faculty members from Auburn’s departments of Chemistry, Physics, and Materials Engineering contributing their expertise. Dr. Miliordos concludes with an optimistic outlook on the future: "This is merely the nascent stage of our exploration. By diligently learning how to harness and manipulate free electrons, we can envision a future characterized by exponentially faster computers, more intelligent and adaptive machines, and the emergence of entirely new technologies that we have yet to even dream of." This forward-looking statement encapsulates the boundless possibilities that this research has unlocked.
The comprehensive study, bearing the title "Electrides with Tunable Electron Delocalization for Applications in Quantum Computing and Catalysis," was further enriched by the insightful contributions of graduate students Andrei Evdokimov and Valentina Nesterova. This pioneering work received crucial support from the U.S. National Science Foundation, underscoring its national significance, and benefited from the extensive computational resources provided by Auburn University, enabling the complex simulations and analyses required for its successful completion. The implications of this research extend far beyond academic curiosity, promising to reshape the technological landscape and redefine the limits of human innovation.