Electrons, the ubiquitous architects of the universe, are the linchpin of virtually every chemical transformation and technological marvel. They orchestrate the flow of energy, forge the bonds that hold matter together, and conduct electricity, forming the bedrock of both intricate chemical syntheses and the sophisticated electronics that define our modern world. In the delicate dance of chemical reactions, electrons are the catalysts for redox processes, the architects of new bonds, and the engines of catalytic activity. In the realm of technology, the judicious management of electron movement and their intricate interactions underpins everything from the microchips powering our devices and the artificial intelligence shaping our future, to the efficient energy conversion in solar cells and the mind-bending potential of quantum computers. Historically, electrons have been largely tethered to the atomic nuclei, their behavior predictable but their full potential constrained. However, a special class of materials, termed electrides, defies this convention, housing electrons that move independently, thereby unlocking a vista of extraordinary new functionalities and applications.
"Our ability to harness and direct these free-roaming electrons opens up a universe of possibilities, allowing us to engineer materials with capabilities that transcend the limitations imposed by nature," explains Dr. Evangelos Miliordos, Associate Professor of Chemistry at Auburn and the senior architect of this seminal study, which was meticulously crafted through advanced computational modeling. This deep dive into the quantum realm has yielded insights that are poised to redefine our material world.
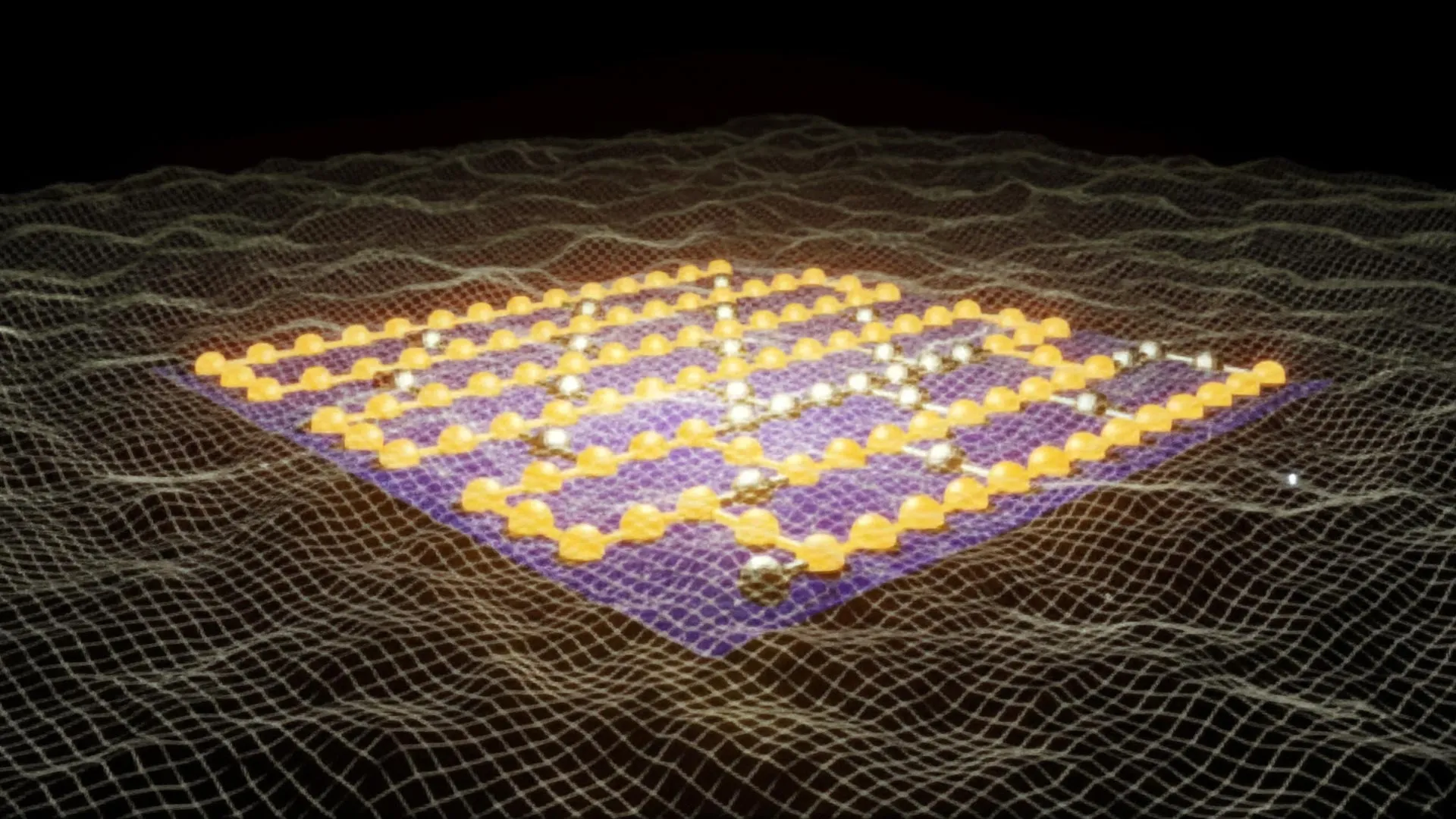
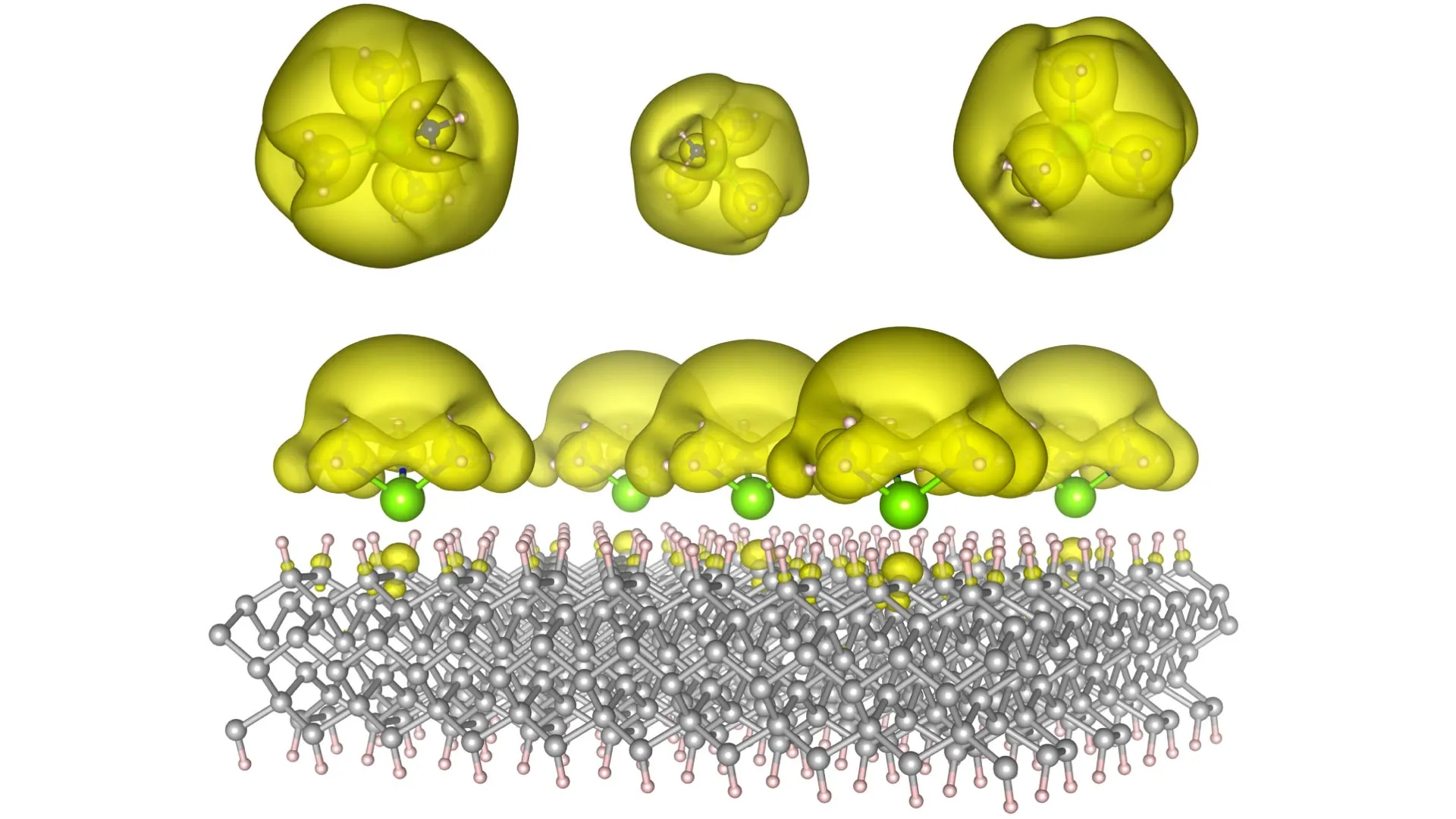
The visionary team at Auburn has ingeniously devised novel material architectures, christened Surface Immobilized Electrides. This innovative approach involves the meticulous attachment of solvated electron precursors onto robust and stable surfaces, such as diamond and silicon carbide. This strategic integration renders the electronic characteristics of these electrides remarkably durable and, crucially, exquisitely tunable. By subtly altering the spatial arrangement of the molecular components, researchers can dictate whether the electrons coalesce into isolated "islands," which exhibit quantum behaviors akin to qubits essential for advanced computing, or whether they spread out into expansive "seas" that actively promote and accelerate complex chemical reactions.
This inherent versatility is the very essence of the discovery’s transformative potential. One iteration of these engineered materials could pave the way for the development of ultra-powerful quantum computers, capable of tackling computational challenges that currently lie far beyond the grasp of even the most sophisticated supercomputers. Simultaneously, another variant could serve as the foundational element for a new generation of cutting-edge catalysts. These catalysts would possess the remarkable ability to dramatically accelerate essential chemical reactions, potentially revolutionizing industries ranging from fuel production and pharmaceutical manufacturing to the creation of novel industrial materials. The ripple effects of such advancements would be felt across the global economy and scientific research.
"As our society relentlessly pushes the boundaries of current technological capabilities, the demand for novel and advanced materials is experiencing an exponential surge," observes Dr. Marcelo Kuroda, Associate Professor of Physics at Auburn. "Our research illuminates a promising new pathway towards the creation of materials that not only offer profound opportunities for fundamental investigations into the intricate interactions within matter but also present tangible and impactful practical applications." This dual promise of fundamental insight and practical utility underscores the significance of their findings.
Previous attempts to synthesize and utilize electrides were plagued by inherent instability and significant challenges in scaling up production. However, by ingeniously depositing these electron-rich precursors directly onto solid surfaces, the Auburn team has effectively surmounted these long-standing hurdles. They have, in essence, proposed a versatile family of material structures that possess the remarkable potential to transition from theoretical constructs and laboratory curiosities to functional, real-world devices. "This is a testament to the power of fundamental science, but its implications are profoundly real and far-reaching," states Dr. Konstantin Klyukin, Assistant Professor of Materials Engineering at Auburn. "We are contemplating technologies that possess the capacity to fundamentally alter how we compute and how we manufacture goods, ushering in an age of unprecedented efficiency and innovation." The potential for disruption and positive change is immense.
The theoretical underpinnings of this groundbreaking study were a collaborative effort, drawing expertise from across the disciplines of chemistry, physics, and materials engineering at Auburn University. This interdisciplinary synergy was crucial in unraveling the complex quantum mechanical principles at play. "This is merely the nascent stage of what we believe will be a revolutionary field," enthusiastically adds Dr. Miliordos. "By dedicating ourselves to understanding and mastering the behavior of free electrons, we can vividly envision a future replete with computers that operate at speeds we can only dream of today, machines that exhibit a level of intelligence and adaptability previously unimaginable, and entirely new technological paradigms that we haven’t even begun to conceive." The journey of discovery is ongoing, with the promise of even greater breakthroughs on the horizon.
The seminal study, aptly titled "Electrides with Tunable Electron Delocalization for Applications in Quantum Computing and Catalysis," was further enriched by the dedicated contributions of graduate students Andrei Evdokimov and Valentina Nesterova, whose meticulous research and analytical skills were instrumental to the project’s success. The ambitious undertaking was made possible through the generous support of the U.S. National Science Foundation, providing vital funding for this cutting-edge research, and the extensive computational resources made available by Auburn University, which were indispensable for conducting the complex simulations and theoretical analyses. This collaborative effort, fueled by academic curiosity and institutional support, has laid the groundwork for a future shaped by quantum materials and the transformative power of precisely controlled electrons.