Electrons, the unsung heroes of the universe, are undeniably central to nearly every chemical reaction and technological process that underpins our modern world. They are the driving force behind energy transfer, the architects of chemical bonding, and the very essence of electrical conductivity, serving as the bedrock for both sophisticated chemical synthesis and the intricate workings of modern electronics. In the intricate dance of chemical reactions, electrons orchestrate redox processes, forge new bonds, and catalyze the transformations that yield everything from life-saving pharmaceuticals to everyday plastics. In the realm of technology, the ability to meticulously manage how electrons move, interact, and are manipulated is fundamental to the operation of everything from the microscopic circuits in our smartphones and the advanced algorithms driving AI systems, to the efficiency of solar cells capturing the sun’s energy and the nascent potential of quantum computers. Historically, electrons have been largely confined to their atomic orbitals, a limitation that has inherently restricted their potential for novel applications. However, in a paradigm shift, materials known as "electrides" offer a radical departure, allowing electrons to move independently, thereby unlocking a Pandora’s Box of remarkable new capabilities.
"By mastering the art of controlling these free-roaming electrons, we unlock the potential to design and engineer materials that can perform functions far beyond what nature has conventionally allowed," explains Dr. Evangelos Miliordos, an Associate Professor of Chemistry at Auburn University and the senior author of this seminal study. The research itself was built upon a foundation of advanced computational modeling, a testament to the synergy between theoretical prediction and experimental realization.
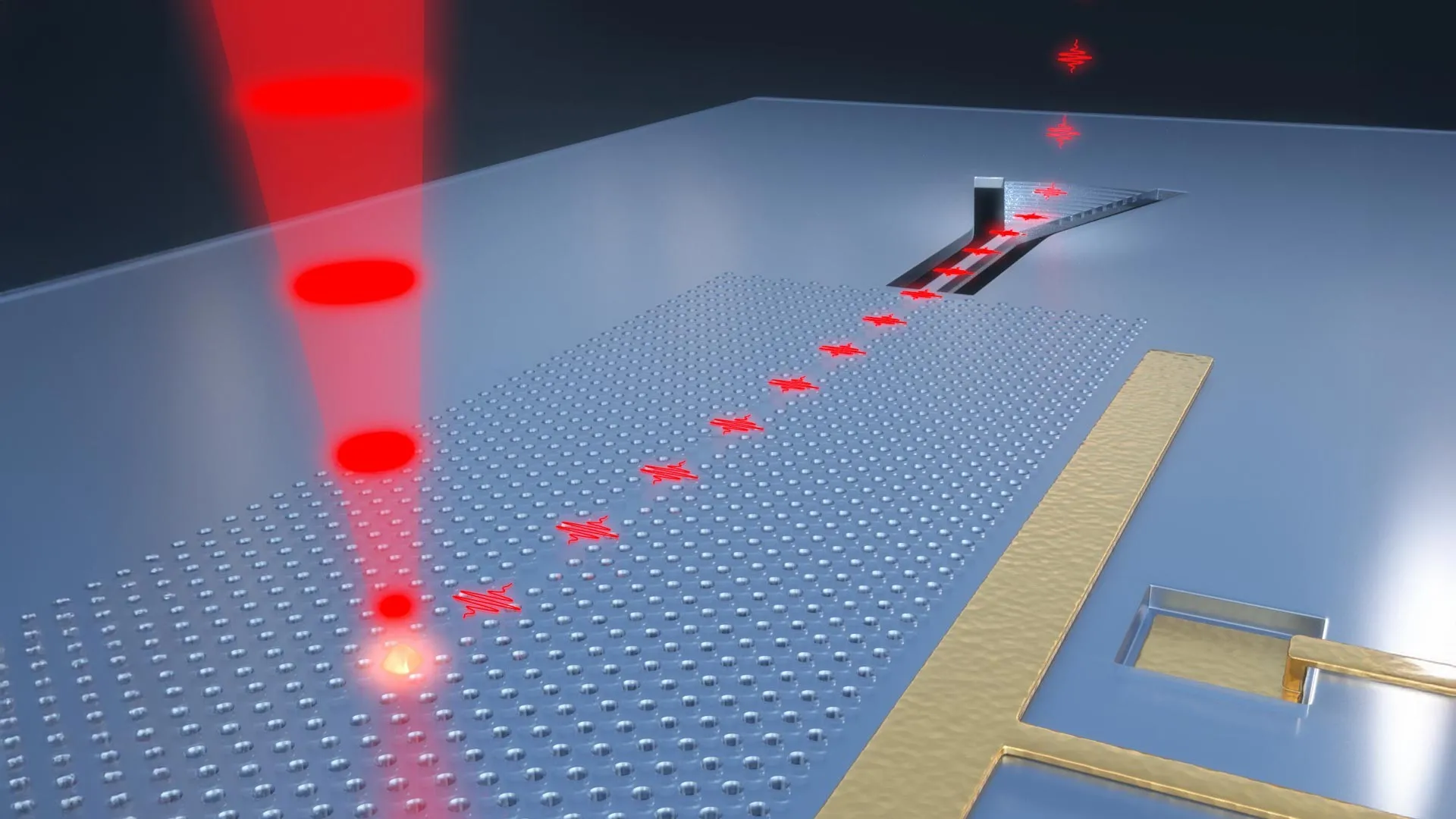
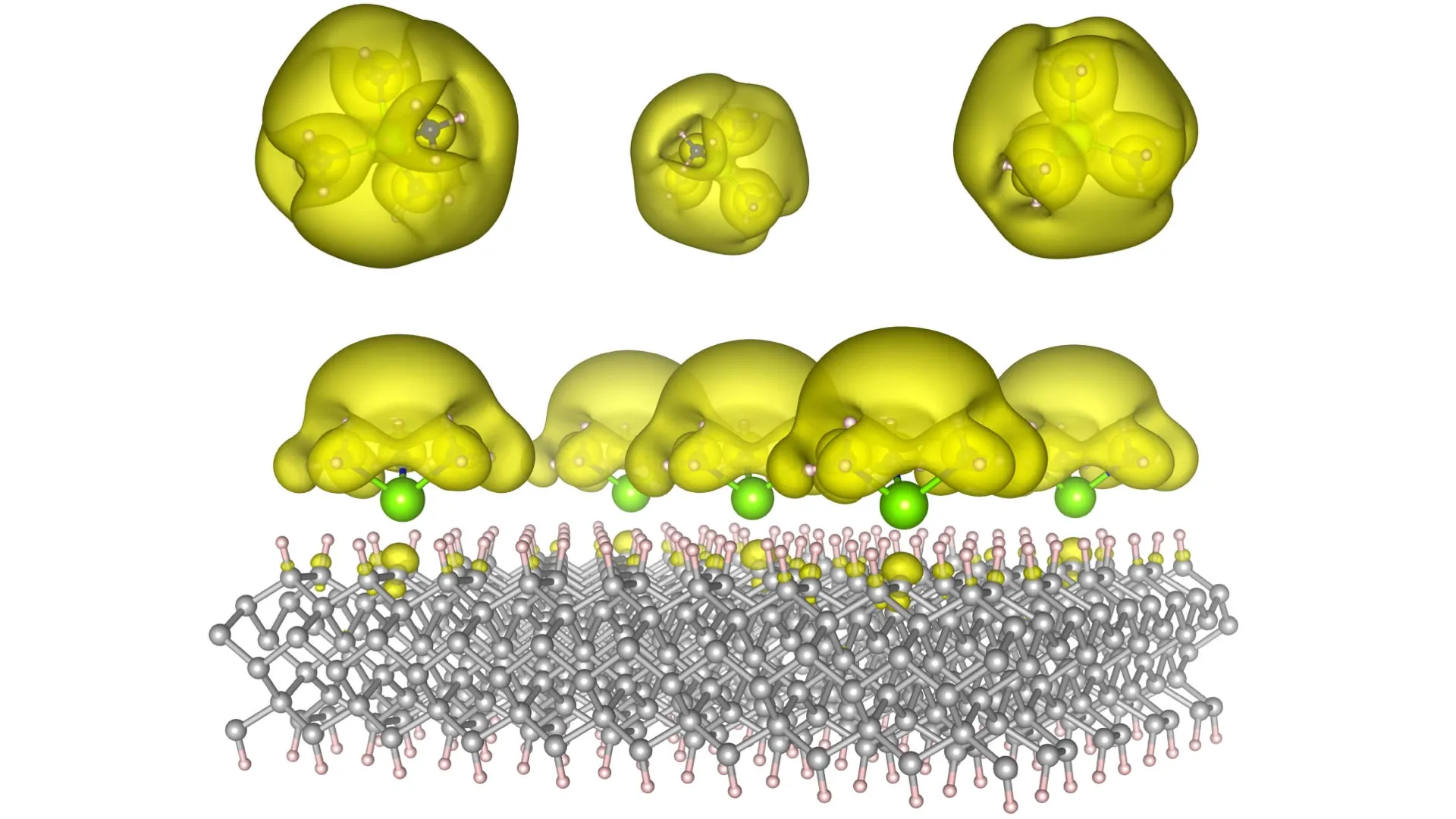
To achieve this unprecedented level of control, the Auburn team conceptualized and fabricated innovative material structures they’ve termed "Surface Immobilized Electrides." This ingenious design involves strategically attaching solvated electron precursors to robust and stable surfaces, such as the highly engineered surfaces of diamond and silicon carbide. This carefully constructed configuration imbues the electrides with electronic characteristics that are not only remarkably durable but also highly tunable. The key to this adjustability lies in the precise manipulation of the molecular arrangement. By altering how these molecules are positioned and interact on the surface, scientists can dictate whether the free electrons cluster into isolated "islands," which behave analogously to quantum bits, or "qubits," the fundamental units of information in quantum computing, or conversely, spread out into extended "seas" of delocalized electrons, a configuration that is ideal for promoting and accelerating complex chemical reactions.
This inherent versatility is precisely what imbues this discovery with its transformative potential. One manifestation of this technology could lead to the development of powerful quantum computers, machines capable of tackling problems that are currently intractable for even the most advanced supercomputers. Imagine drug discovery accelerated by simulating molecular interactions with unprecedented accuracy, or complex climate models offering insights into global warming with remarkable precision. Another facet of this discovery could lay the groundwork for cutting-edge catalysts, substances that dramatically speed up essential chemical reactions. This could revolutionize industries ranging from energy production, by enabling more efficient fuel synthesis, to the pharmaceutical sector, by streamlining the creation of life-saving medicines, and the manufacturing of advanced industrial materials.
"As our society relentlessly pushes the boundaries of current technological capabilities, the demand for novel and sophisticated materials is experiencing an explosive surge," observes Dr. Marcelo Kuroda, an Associate Professor of Physics at Auburn University. "Our research presents a completely new pathway to developing materials that not only offer profound opportunities for fundamental investigations into the intricate interactions within matter but also hold immense promise for immediate and practical real-world applications."
Previous attempts to harness the power of electrides were often hampered by their inherent instability and the significant challenges associated with scaling up their production. By ingeniously depositing these electron-rich precursors directly onto solid surfaces, the Auburn team has effectively overcome these critical barriers. They have not only demonstrated the feasibility of creating these materials but have also proposed a versatile family of material structures that can bridge the gap between theoretical models and tangible, functional devices. "This is fundamental science at its most profound, but its implications are extraordinarily real," emphasizes Dr. Konstantin Klyukin, an Assistant Professor of Materials Engineering at Auburn University. "We are on the cusp of developing technologies that have the potential to fundamentally alter the way we compute and the way we manufacture goods, ushering in an era of unprecedented innovation."
The theoretical underpinnings of this groundbreaking study were meticulously developed through a collaborative effort involving faculty members from diverse disciplines across chemistry, physics, and materials engineering at Auburn University. "This is merely the nascent stage of what we believe will be a revolutionary field," Dr. Miliordos adds with evident enthusiasm. "By developing a profound understanding of how to harness and precisely control free electrons, we can begin to envision a future characterized by significantly faster and more powerful computers, exceptionally intelligent machines, and entirely new categories of technologies that we haven’t even begun to conceive of yet."
The comprehensive study, titled "Electrides with Tunable Electron Delocalization for Applications in Quantum Computing and Catalysis," was further enriched by the contributions of graduate students Andrei Evdokimov and Valentina Nesterova, who played crucial roles in the research and data analysis. The project received vital support from the U.S. National Science Foundation, providing essential funding for fundamental research, and from Auburn University’s advanced computing resources, which were indispensable for the sophisticated modeling and simulations required for this work. The discovery of Surface Immobilized Electrides represents a significant leap forward in materials science, promising to unlock new frontiers in technological innovation and scientific understanding.