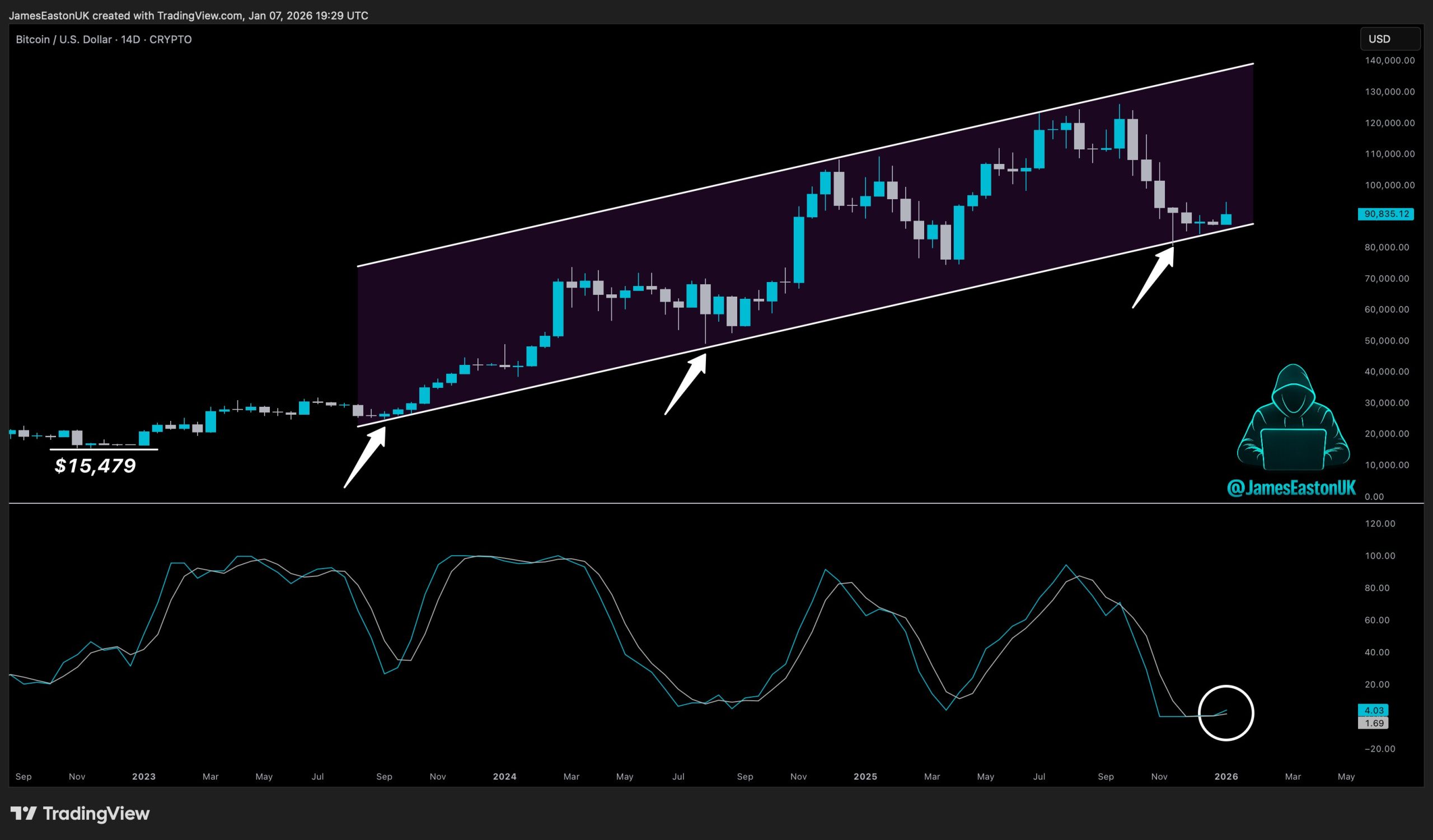
The once-heralded CRISPR gene-editing technology, lauded by MIT Technology Review as the biggest biotech breakthrough of the century since 2013, is facing a reality check. Despite its immense potential, only one gene-editing drug has received regulatory approval to date, impacting a mere 40 patients, all suffering from sickle-cell disease. This limited commercialization has cast a shadow of discouragement over the field, with some observers questioning whether the gene-editing revolution has “lost its mojo,” as highlighted by STAT News.
In response to this slowdown, a new startup, Aurora Therapeutics, is pioneering an innovative "umbrella approach" to streamline the testing and commercialization of gene-editing treatments. Backed by $16 million in funding from Menlo Ventures and advised by CRISPR co-inventor Jennifer Doudna, Aurora aims to secure approvals for gene-editing drugs that can be readily adapted for individual patients without requiring entirely new, costly clinical trials and regulatory reviews for each iteration.
This strategic pivot aligns with growing recognition from regulatory bodies. In November, Martin Makary, head of the US Food and Drug Administration (FDA), publicly endorsed the necessity for updated regulations, signaling the agency’s intent to establish a "new" regulatory pathway for "bespoke, personalized therapies" that defy conventional testing methodologies.
Aurora’s initial focus is on phenylketonuria (PKU), a rare inherited metabolic disorder. Individuals with PKU lack a functional enzyme crucial for processing the amino acid phenylalanine, found abundantly in protein-rich foods. A buildup of phenylalanine can lead to severe neurological damage, necessitating a lifelong, restrictive diet of specialized formulas and limited food choices.
While gene editing holds theoretical promise for correcting PKU, the genetic landscape of the disease presents a significant challenge. Research in mice has demonstrated the potential to restore the defective enzyme gene by editing DNA in liver cells, which are both primary producers of the enzyme and relatively accessible targets for gene-editing therapies. However, the complexity arises from the vast genetic variability in human PKU patients. According to Cory Harding, a researcher at Oregon Health Sciences University, scientists have identified approximately 1,600 distinct DNA mutations that can cause PKU. Developing 1,600 separate gene-editing drugs is an impractical endeavor.
Aurora’s ambitious goal is to obtain approval for a single gene editor capable of correcting several of the most prevalent mutations through minor adjustments. This approach could address a significant portion of the estimated 20,000 PKU cases in the US, including a mutation responsible for about 10% of them. Edward Kaye, CEO of Aurora, emphasizes the regulatory bottleneck: "We can’t have a separate [clinical trial] for each mutation. The way the FDA approves gene editing has to change, and I think they’ve been very understanding that is the case."
The core of Aurora’s technology lies in its gene editor, a specialized protein designed to precisely target and modify specific genomic locations. To deliver this editor, Aurora will package its genetic code into a nanoparticle, alongside a targeting molecule. The entire delivery system will comprise roughly 5,000 genetic letters, with only about 20 needing modification to redirect the treatment towards a different mutation. Johnny Hu, a partner at Menlo Ventures, highlights the efficiency of this method, stating, "Over 99% of the drug stays the same."
The genesis of Aurora Therapeutics can be traced to a conversation between Johnny Hu and Fyodor Urnov, a prominent gene-editing scientist at the University of California, Berkeley, and a co-founder and board member of Aurora. Urnov, known for his outspoken views on the field, had previously articulated in a New York Times editorial the "chasm" between the technological capabilities of gene editing and the "legal, financial, and organizational" realities hindering its widespread application for curing diseases.
Hu approached Urnov with a critical question: "Hey, we’re getting all these great results in the clinic with CRISPR, but why hasn’t it scaled?" He identified a key factor: many gene-editing companies are pursuing the same few conditions, like sickle-cell disease, where a single genetic edit suffices for all patients. This leaves an estimated 400 million individuals with 7,000 other inherited conditions with limited hope for genetic therapies, as Urnov pointed out.
A pivotal event in this evolving landscape was the recent demonstration of the first fully "personalized" gene-editing treatment. In May, a team in Philadelphia, with contributions from Urnov and others, successfully corrected a unique mutation in a baby named KJ Muldoon, who suffered from a rare metabolic disease. Although this case did not involve PKU, it powerfully illustrated the potential of gene editing to address inherited diseases "on demand."
However, this breakthrough also exposed a significant challenge. The treatment for KJ, while groundbreaking, required a substantial team, immense effort, and millions of dollars, yielding a drug that would likely never be replicated for another patient. This is precisely the scenario that Aurora’s "umbrella" trial approach aims to circumvent.
Kiran Musunuru, who co-led the team treating Baby KJ at the University of Pennsylvania, is engaged in discussions with the FDA to launch a study of bespoke gene editors focused on diseases similar to KJ’s, known as urea cycle disorders. He envisions a rapid development process where, for each new patient, a tailored variant of the gene-editing drug would be quickly assembled to address their specific genetic defect.
Musunuru, while not directly involved with Aurora, differentiates their PKU strategy from the "fully personalized" editors demonstrated in KJ’s case. He states, "These corporate PKU efforts have nothing whatsoever to do with Baby KJ," explaining that his center focuses on mutations so exceptionally rare that they are unlikely to be commercially viable for for-profit gene-editing companies. Instead, Musunuru characterizes the PKU approach as a strategic aggregation of the most common mutations into a group large enough to support a commercially viable platform therapy.
While this platform approach may not benefit patients with ultra-rare gene errors, Musunuru acknowledges that any gene-editing treatment for PKU would represent "a big improvement over the status quo, which is zero genetic therapies for PKU." This sentiment underscores the broader ambition of Aurora Therapeutics: to unlock the transformative potential of CRISPR by navigating and reshaping the regulatory landscape to bring these life-changing therapies to a wider patient population.